

AffiVET® Swine Fever Virus Antigen Rapid Test
CAT#: AFG-VGS-65
Size: 40 Tests
Method: Rapid Test
Species: Swine
Specimen: Serum & Whole Blood
Test Principal:
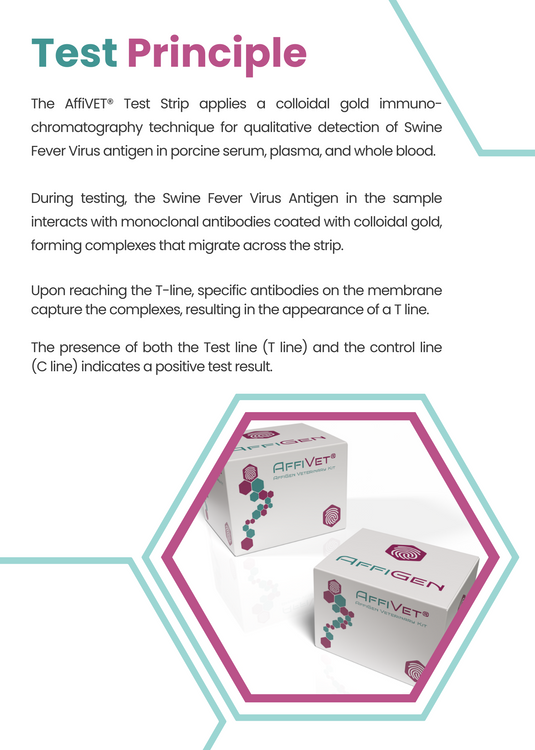
- The AffiVET® Test Strip applies a colloidal gold immuno-chromatography technique for qualitative detection of Swine Fever Virus antigen in porcine serum, plasma, and whole blood.
- During testing, the Swine Fever Virus Antigen in the sample interacts with monoclonal antibodies coated with colloidal gold, forming complexes that migrate across the strip.
- Upon reaching the T-line, specific antibodies on the membrane capture the complexes, resulting in the appearance of a T line.
- The presence of both the Test line (T line) and the control line (C line) indicates a positive test result.
Material Required:
(40) CSFV Antigen Test cards
(40) Droppers
(01) User Manual
Storage & Expiry Date :
1) Store the test kit at 4 to 30°C in dark, sealed, dry place. DO NOT FREEZE.
2) The expiry date is 12 months from the production date, which is indicated on the box.
Sample Collection & Preparation:
Whole Blood:
- Collect anticoagulated whole blood.
- Test the same day.
Serum:
- Collect blood and keep at 37°C for 1-2h.
- Take the supernatant and centrifuge at 1500r/min for 10min then separate the serum.
- The serum can be stored for 2-3 days at 4°C.
- For long term storage, freeze at -20°C.
Test Procedure:
- Read the manual carefully.
- The test card and the sample need to be at room temperature before use.
- Take out the test card.
- Use the dropper to absorb the sample.
- Drop 2 to 3 drops into the well marked with “S”, or use Micropipette to transfer 70 µL of the sample into well mark "S".
- Read the results in 5 to 10 min at room temperature.
- The result is invalid after 15 min.
Result Interpretation:
Negative Result:
- Presence of C-Line
- Absence of T-Line
This means there is no Swine Fever Virus Antigen in sample or the level is lower than the detection threshold.
Positive Result:
- Presence of C-Line
- Presence of T-Line
This confirms the presence of the Swine Fever Virus Antigen in sample.
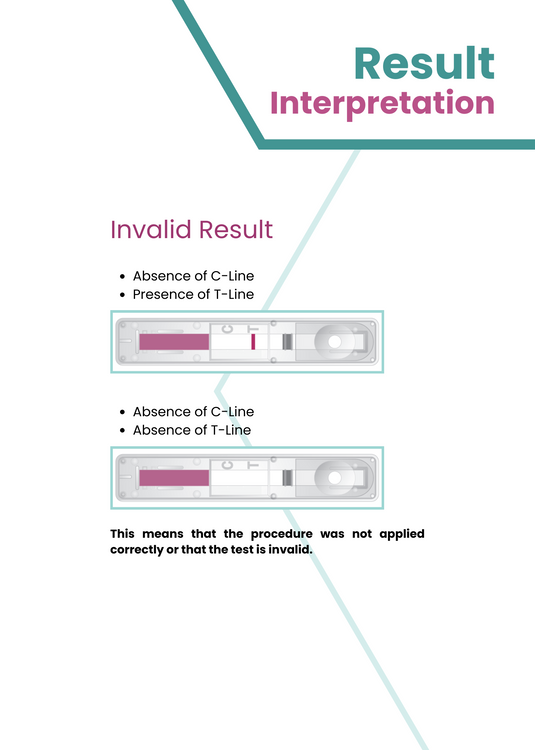
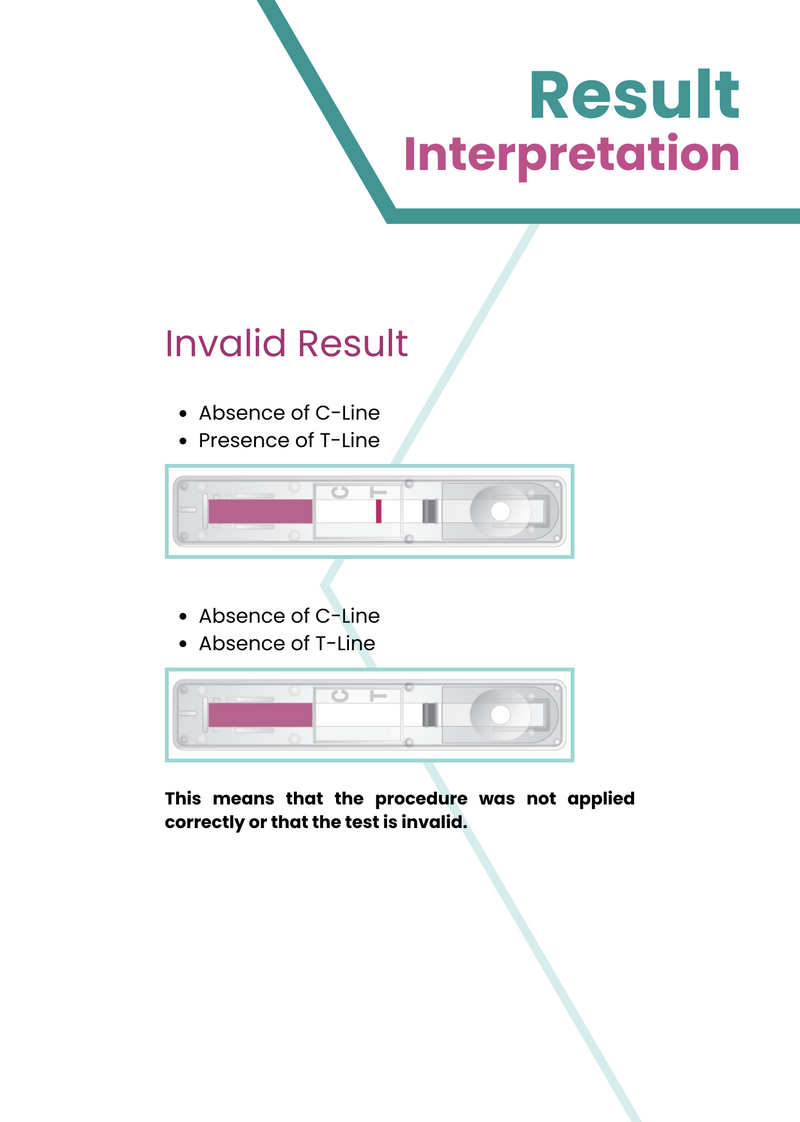
Invalid Result:
- Absence of C-Line
- Presence of T-Line
or
- Absence of C-Line
- Absence of T-Line
This means that the procedure was not applied correctly or that the test is invalid.
Cautions:
- The test card can only be used when brought to room temperature.
- Don’t reuse the test card or use it after the expiry date.
- Bring the sample and all the reagents to room temperature before use.
- When the test card is unsealed, please use it as soon as possible.
- Take precaution while preparing the sample. Gloves and mask are required.
- The whole blood needs to be collected in anticoagulant tube.
- Don’t freeze the whole blood.
- The serum sample can be stored frozen but avoid repeated freezing and thawing.
- This product is only for in vitro rapid diagnostic veterinary use.
- This is a qualitative test. It is not quantitative.
- The test results are for reference only. For confirmation, please refer to the relevant national standard methods.