AffiVET® Camel MERS CoV Antigen Rapid Test Kit
Intended Use:
Qualitative detection of Middle East Respiratory Syndrome coronavirus (MERS-CoV) antigen in camel nasal/oral secretions.
Principle of the Assay:
Type: Lateral flow immunochromatographic assay.
Mechanism:
- Sample added to cassette → migrates by capillary action.
- If MERS-CoV antigens are present → form complex with pre-coated antibodies.
- Colored T line (test) appears → positive.
- C line (control) must always appear to confirm valid test.
Storage and Stability:
- Temperature: 2–30 °C (room temperature or refrigerated).
- Do not freeze.
- Stable until expiry date printed on pouch.
- Keep sealed until use.
Precautions:
- Do not use after expiration or if pouch is damaged.
- Treat all specimens as potentially infectious.
- Use gloves and eye protection.
- Humidity and temperature may affect results.
- Do not reuse test components or mix lots.
- Single-use only.
Kit Components:
- Test Cassettes ------------------------ (10 Devices)
- Disposable Droppers ---------------- (10 Pieces)
- Assay Diluents ------------------------ (10 Pieces)
- Disposable Swabs -------------------- (10 Pieces)
- Instruction Manual ------------------- (1 Piece)
Required but not provided: Timer
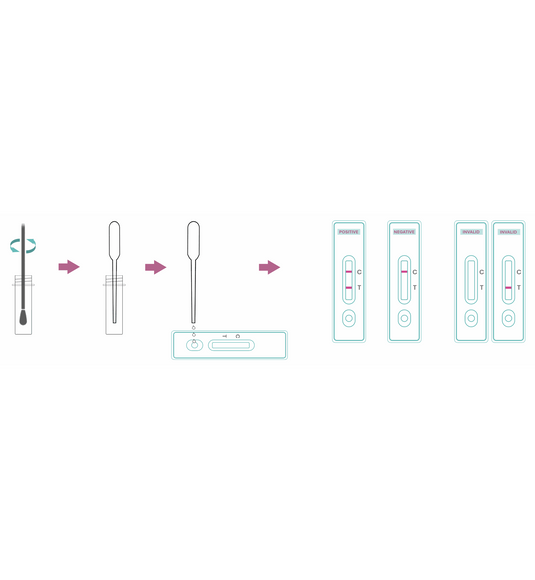
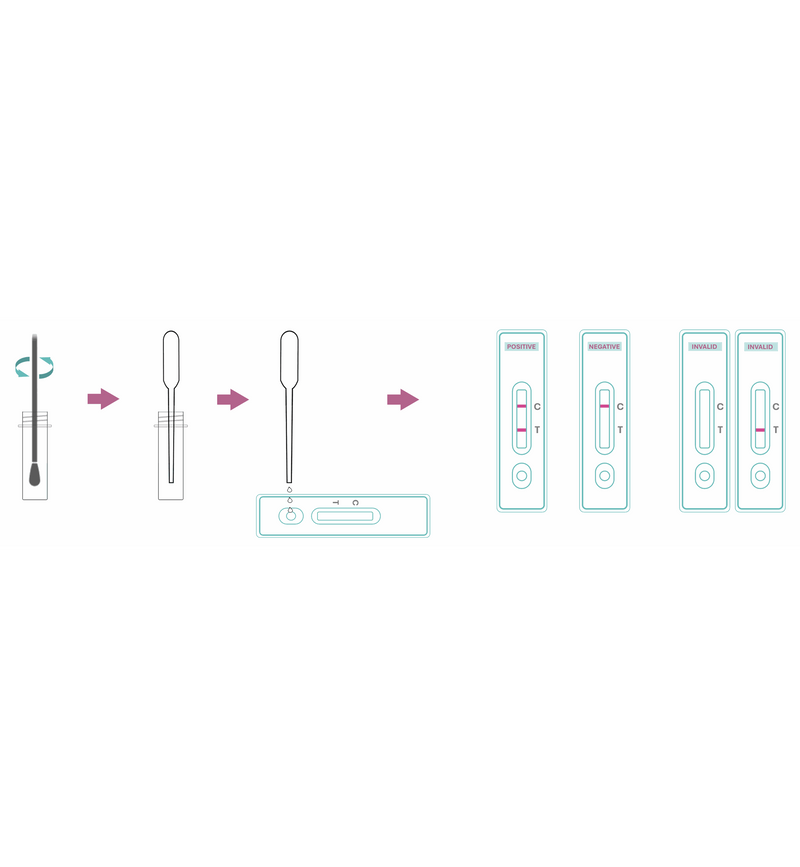
Test Procedure:
- Bring cassette, specimen, and buffer to 15–30 °C.
- Open pouch and use cassette within 1 hour.
- Collect sample: nasal/oral secretion using provided swab (ensure sufficient wetting).
- Insert swab into buffer tube and mix thoroughly.
- Using dropper, add 3 drops (~120 µL) of extracted sample to sample well (S).
- Start timer and read at 15 minutes (ignore results after 25 minutes).
Interpretation of Results:
- Positive: Both C and T lines visible (T may be faint or strong). [MERS-CoV antigen detected].
- Negative: Only C line visible. [No MERS-CoV antigen detected].
- Invalid: No C line visible. [Test failed – repeat].

Limitations:
- For camel application only.
- Results should be interpreted with clinical context.
- Confirmatory testing (e.g. PCR) recommended for verification.
Validation Data:
- Sensitivity > 95%
- Specificity > 95%
- Accuracy > 95%
Cross Reactivity:
The test was evaluated using samples positive for other viruses including Coronavirus, SARS-CoV, Influenza A, Influenza B, and Camel Pox.
No cross-reactivity observed with these pathogens.