AffiGEN Blog News | AffiGEN Inc.
-

AffiELISA® CLIC1, CLIC2, CLIC3, CLIC4, CLIC5, and CLIC6 ELISA Kits
Overview Chloride intracellular channel proteins (CLICs) are a unique family of multifunctional proteins found in various tissues and cellular compartments. Initially, CLICs were considered to be solely involved in chloride... -

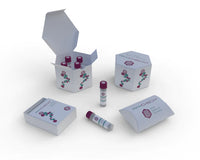
AFFICHECK® Strep A PCR Controls
AffiCHECK®Strep A PCR Controls Positive Control Negative Control External Run Control Panel Control Verified Platforms Cepheid® GeneXpert® Luminex® Aries® AFG-CHK-0405 | AffiCHECK® Streptococcus A PCR... -

FeLV Feline Leukemia Virus
What is Feline Leukemia Virus ? Feline leukemia virus affected cats can develop anemia (a low red blood cell level), cancers, and/or suppression of the immune system. The disease worsens... -
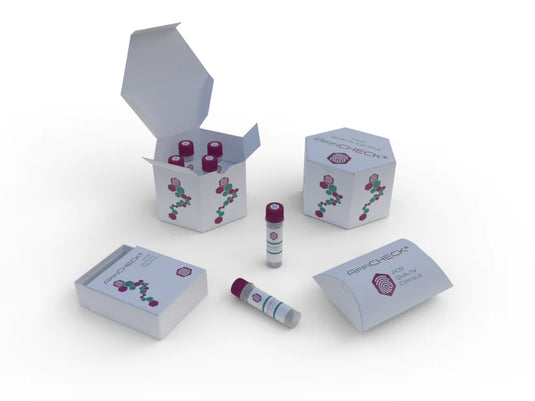
AffiCHECK® PCR Controls by Equipment - Enhancing Diagnostic PCR Accuracy
Improve the accuracy of diagnostic PCR with AffiCHECK® PCR Controls by Equipment. Developed by AffiGEN®, a trusted provider of molecular biology solutions, these controls are designed to optimize PCR performance on various diagnostic equipment. -
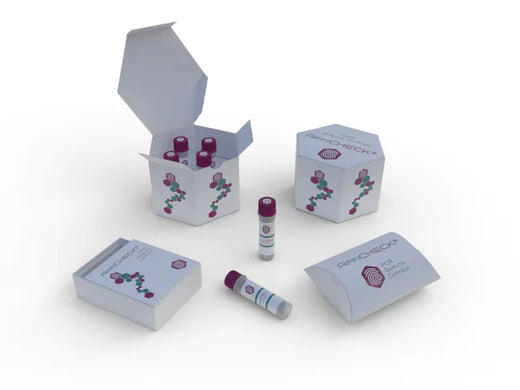
AffiCHECK® PCR Controls by Pathogen
At AffiGEN®, we understand the critical role of PCR controls in ensuring the validity of your molecular biology experiments. AffiCHECK® PCR Controls specifically developed to validate and monitor PCR assays, allowing you to detect and amplify target sequences with utmost precision. Let's explore the classification of pathogens tested by PCR and the comprehensive coverage provided by AffiCHECK® PCR Controls. -

Quantify Human IL-1 beta Levels with High Precision using the Human IL-1 beta ELISA Kit
The Human IL-1 beta ELISA Kit is a powerful tool for researchers seeking to investigate the role of IL-1 beta in various physiological and pathological processes. This article provides a comprehensive overview of the assay protocol, highlighting the step-by-step instructions for sample preparation, plate setup, antibody incubation, washing, substrate addition, and data analysis. Furthermore, it delves into the wide range of applications where the Human IL-1 beta ELISA Kit can be employed, such as studying inflammation, immune responses, and related diseases. With its high sensitivity and specificity, this kit enables accurate quantification of IL-1 beta levels in human samples, providing valuable insights into the underlying mechanisms of various conditions. Discover the benefits of incorporating the Human IL-1 beta ELISA Kit into your research arsenal and unlock new avenues for understanding and targeting IL-1 beta-mediated pathways. -
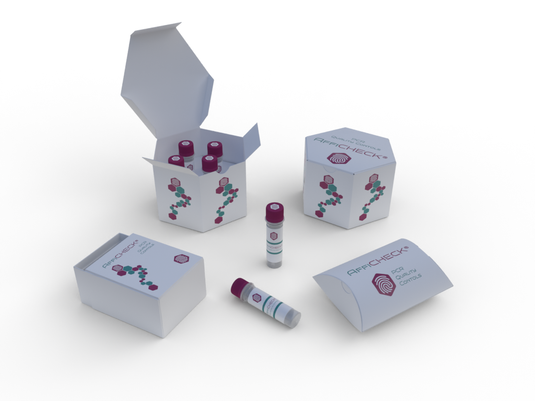
Schistosoma spp. (Parasite) PCR Run Control: Enhancing Accuracy and Reliability of Diagnostic Testing
The detection of Schistosoma spp., a parasitic infection causing significant morbidity and mortality worldwide, relies on sensitive and specific diagnostic methods. Polymerase chain reaction (PCR) assays have emerged as a valuable tool for the detection of Schistosoma spp. DNA in clinical and environmental samples. However, to ensure the reliability and accuracy of PCR results, the inclusion of a PCR run control is essential. This article provides a comprehensive overview of the technical aspects, general lab protocol, and detailed applications of the Schistosoma spp. PCR Run Control. It highlights its role in diagnostic testing, research studies, surveillance programs, quality assurance, and training. By implementing the PCR run control, laboratories can enhance the robustness of their PCR assays and contribute to improved diagnosis, monitoring, and control of Schistosoma spp. infections. -
Trichomonas vaginalis PCR Run Control: Enhancing Accuracy and Reliability in Trichomoniasis Diagnosis
Trichomonas vaginalis PCR Run control plays a crucial role in enhancing the accuracy of diagnostic testing for Trichomonas vaginalis, a sexually transmitted parasite. By including a run control in PCR assays, laboratories can ensure the reliability and validity of their test results.
The Trichomonas vaginalis PCR Run control is designed to mimic the target DNA sequence of the parasite and is added to the PCR reaction alongside patient samples. This control serves as a benchmark for the performance of the PCR assay, allowing for the detection of any potential issues or errors in the test.
The run control helps to monitor various aspects of the PCR process, including DNA extraction, amplification, and detection. It provides a reference point for evaluating the efficiency and sensitivity of the assay, as well as identifying any factors that may impact the accuracy of the test.
In addition to validating the PCR assay, the Trichomonas vaginalis PCR Run control also serves as a quality control measure. It helps to ensure the consistency and reliability of test results across different runs or batches, minimizing the risk of false-negative or false-positive results.
Overall, the inclusion of a Trichomonas vaginalis PCR Run control in diagnostic testing protocols is essential for maintaining the accuracy and reliability of results. It enables laboratories to identify and address any potential issues, improving the overall quality of Trichomonas vaginalis detection and diagnosis.
-
Giardia lamblia PCR Run Control: Enhancing Accuracy and Reliability in Molecular Diagnostic Assays
The use of PCR Run controls is crucial in ensuring the accuracy and reliability of PCR assays for the detection of Giardia lamblia, a parasitic protozoan that causes gastrointestinal infections in humans. These controls consist of positive and negative samples that are run alongside the test samples to validate the PCR assay's performance.
The positive control samples contain known concentrations of Giardia lamblia DNA or cultured organisms, providing a reference for expected amplification. By including positive controls in each PCR run, laboratories can assess the sensitivity and specificity of the assay and monitor for potential issues such as PCR inhibition or equipment malfunction.
On the other hand, negative control samples are essential for assessing the specificity of the PCR assay and confirming the absence of cross-reactivity or contamination. These negative controls should produce no amplification signal, ensuring that false-positive results are minimized.
By implementing a well-designed and standardized protocol for Giardia lamblia PCR Run control, laboratories can validate the performance of their PCR assays, maintain quality assurance, and ensure accurate detection of Giardia lamblia infections.
This is an excerpt from a larger technical article on Giardia lamblia PCR Run control.
-
Plasmodium falciparum PCR Run Control: Assuring Quality in Malaria Diagnostics
Plasmodium falciparum PCR Run control is an essential component of molecular diagnostic assays targeting the detection and identification of P. falciparum, the causative agent of malaria. This control material serves as a reference sample to ensure the accuracy and reliability of the PCR assay by assessing the performance of the amplification reaction.
The P. falciparum PCR Run control consists of either synthetic control material or extracted DNA from cultured P. falciparum parasites. It contains specific target sequences that mimic the genomic DNA of the parasite. By including this control in each PCR run, laboratories can monitor the efficiency of the amplification process, identify potential issues or variations, and validate the overall performance of the assay.
During the PCR assay setup, the P. falciparum PCR Run control is added to designated reaction tubes or wells along with the PCR master mix. The amplification is then carried out using the appropriate cycling conditions. After PCR amplification, the presence or absence of the expected P. falciparum amplification signal is analyzed using gel electrophoresis or real-time PCR instrumentation.
The P. falciparum PCR Run control is particularly useful in validating the accuracy of diagnostic assays, assessing the sensitivity and specificity of the test, and identifying potential PCR inhibitors or technical issues that may affect the assay results. It also aids in the interpretation of patient samples by providing a reliable reference for comparison.
In conclusion, the P. falciparum PCR Run control is an integral part of molecular diagnostic assays targeting P. falciparum detection. Its inclusion in each PCR run allows for quality control, assurance of accurate results, and validation of the overall performance of the assay.
-
Toxoplasma gondii PCR Run Control: Ensuring Accurate Detection of Parasitic Infection
Toxoplasma gondii PCR Run Control is a crucial component in the accurate detection and monitoring of T. gondii infections using PCR assays. This control is designed to mimic the target DNA sequence of T. gondii and serves as a positive control during PCR amplification. By including the PCR Run Control in each assay, laboratories can validate the performance of the PCR reaction, monitor the efficiency of the amplification process, and ensure reliable and consistent results.
The Toxoplasma gondii PCR Run Control is typically available as synthetic DNA fragments or purified T. gondii DNA. The control should be stored properly to maintain its stability and integrity. It is recommended to aliquot the control into smaller working volumes and store them at an appropriate temperature, such as -20°C, to avoid degradation and contamination.
During the PCR assay setup, the Toxoplasma gondii PCR Run Control is added to the reaction tubes or plates along with the PCR master mix and other necessary components. The concentration of the control should be within the recommended range to ensure accurate assessment of the PCR performance.
After PCR amplification, the products are typically analyzed using agarose gel electrophoresis. The presence of a specific amplification band of the expected size indicates a valid PCR run, while absence of amplification or deviation from the expected size may indicate issues with the PCR process.
The use of Toxoplasma gondii PCR Run Control is crucial for quality assurance in T. gondii PCR testing. It helps to verify the reliability and reproducibility of the PCR assay, detect potential false negatives or positives, and ensure the accuracy of T. gondii detection in clinical samples.
In summary, the Toxoplasma gondii PCR Run Control is an essential tool in molecular diagnostic laboratories performing PCR-based detection of T. gondii. It allows for reliable and standardized assessment of the PCR assay's performance, leading to accurate and clinically meaningful results.
-
Cryptococcus neoformans PCR Run Control: Enhancing Accuracy in Fungal Detection
In the field of molecular diagnostics, Cryptococcus neoformans PCR Run Control plays a crucial role in ensuring reliable detection of this pathogenic fungus. This technical article provides an in-depth understanding of the principles and applications of Cryptococcus neoformans PCR Run Control. It discusses the lab protocol for implementing this control, highlighting the steps involved in running the PCR assay and interpreting the results. By maintaining the integrity of the testing process and providing quality assurance, Cryptococcus neoformans PCR Run Control enhances the accuracy and reliability of fungal detection in clinical and research settings.